
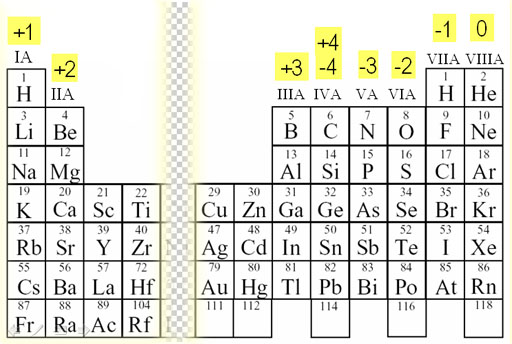
There isn't a shell below the first electron shell so it has no core electrons. All other electrons would be considered core electrons.įor helium it only has two 1s electrons which constitute the valence shell. For noble gases the valence electrons are the s and p electrons of the highest electron shell. The core electrons for any atom are all the electrons of the atom which aren't valence electrons. Hope that starts to clarify things a little more. Electrons are always partially in the nucleus. All electron states overlap with the nucleus, so the concept of an electron "crashing into" the nucleus does not really make sense. The states with more energy are more spread out. An electron in an atom spreads out according to its energy.
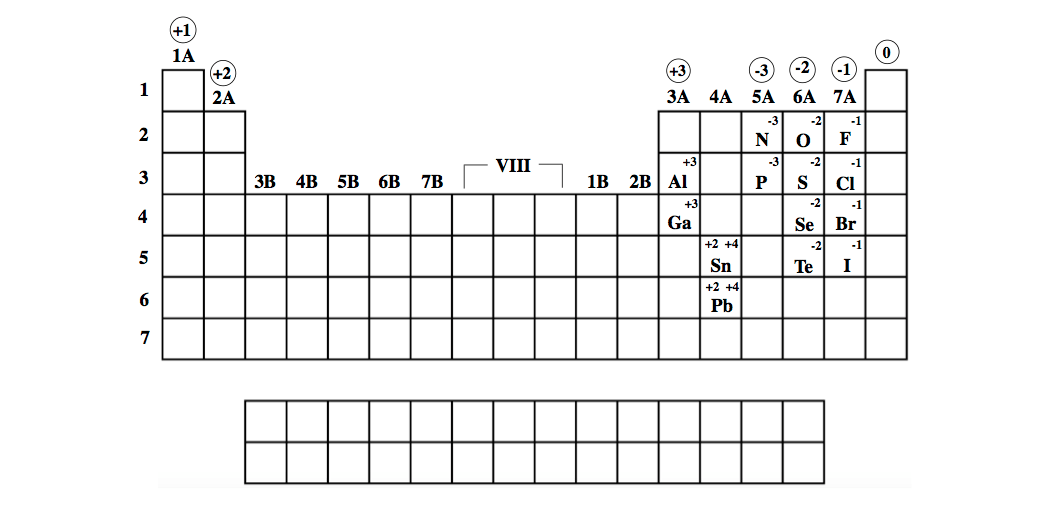
In fact, electrons in the s orbitals tend to peak at the nucleus. And using this understanding of the electron, electrons in the atom do enter the nucleus. Under quantum mechanics the electron is a quantized wave function which occupies certain probable regions around the nucleus called orbitals. To accurately describe the nature of electrons, we must resort to quantum mechanics. This is using classical mechanics to describe electrons when in reality they behave much more differently. So the issue with thinking electrons could crash into the nucleus is that it assumes electrons are solid particles orbiting the nucleus according to Coulomb's Law much like how planets orbit the sun because of gravity.


 0 kommentar(er)
0 kommentar(er)
